Ionic Equation of Magnesium and Hydrochloric Acid
Write the ionic equation by breaking all the soluble ionic compounds those marked with an aq into their respective ions. The flammability of hydrogen gas can be demonstrated by carefully holding a match or fireplace.

Oxidation Magnesium Oxygen Magnesium Oxide Ppt Download
What is the equation for magnesium and hydrochloric acid.

. What is the ionic equation for MG and HCl. Mg s 2 HCl aq --MgCl2 aq H2 g This demonstration can be used to illustrate the characteristic reaction of metals with acid a single replacement reaction or to. Ionic equation of magnesium added to dilute hydrochloric acid.
For the reaction of magnesium with hydrochloric acid is. Derek Answeregy Expert. Magnesium reacts with hydrochloric acid according to the equation.
HI Hydroiodic acid H-aq Iaq HBr Hydrobromic acid H-aq Braq HCl Hydrochloric acid H-aq Cl aq HNO 33 Nitric acid H-aq NO aq HClO. Magnesium reacts with hydrochloric acid according to the equation. Each ion should be shown with its charge and an aq to show that it is present in solution.
For the total ionic equations write strong electrolytes in solution in the form of aqueous ions. Mg s 2 HCl aq â MgCl 2 aq H 2 g This demonstration can be used to illustrate the characteristic reaction of metals with acid a single substitution reaction or to demonstrate the generation of hydrogen gas. Write the ionic equation for the acid-carbonate reaction between hydrochloric acid and insoluble magnesium carbonate to form magnesium chloride salt water and carbon dioxide.
This reaction is an example of a single replacement. H e- rarr 12H_2g And thus the net ionic equation is Mgs 2H rarr Mg2 H_2guarr Overall. Provide the ionic equation.
For example the ionic equation. Identify the general reaction category. Provide the molecular equation 2.
Below is the reaction of a solution of magnesium hydroxide and hydrochloric acid. What is the ionic equation for magnesium and hydrochloric acid. For the reaction of magnesium with hydrochloric acid is.
Magnesium reacts with hydrochloric acid according to the equation. If the mixing results in a combination of ions that forms an insoluble compound a solid may form. Mgs 2 HClaq -- MgCl 2 aq H 2 g This demonstration can be used to illustrate the characteristic reaction of metals with acid a single replacement reaction or to demonstrate the generation of hydrogen gas.
The reaction between magnesium and hydrochloric acid combine to form a salt of magnesium chloride and release hydrogen gas. The solubility rules are useful in predicting what will happen if solutions of two different soluble compounds are mixed. Mgs rarr Mg2 2e- Protium ion is REDUCED to dihydrogen gas.
For example the ionic equation. MgOH₂ 2 HCl - MgCl₂ 2 H₂O. Magnesium reacts with hydrochloric acid according to the equation.
This type of reaction is called a precipitation. The equation for the reaction is. 2H aq Mgs Mg 2 aq H 2 g This ionic equation can be split into two half equations.
What is the ionic equation for magnesium and hydrochloric acid. Mgs 2 HClaq MgCl 2 aq H 2 g This demonstration can be used to illustrate the characteristic reaction of metals with acid a single replacement reaction or to demonstrate the generation of hydrogen gas. Mgs 2HClaq MgCl 2 aq H 2 g Explanation.
The balanced chemical equation is. Use coefficients to show the number of each ion present. Net Ionic Equation For Perchloric Acid And Potassium Hydroxide.
The common strong acids and their aqueous ions are. Magnesium hydrochloric acid magnesium chloride hydrogen Mg s 2HCl aq MgCl 2 aq H 2 g Students follow the rate of reaction between magnesium and the acid by measuring the amount of gas produced at 10 second intervals. Reacting a lead II nitrate Solution with a Potassium iodide Solution 1.
Mg s 2 HCl aq MgCl 2 aq H 2 g 3. 2H aq Mgs Mg 2 aq H 2 g This ionic equation can be split into two half equations. Magnesium reacts with hydrochloric acid according to the equation.
There are three main steps for writing the net ionic equation for Mg HCl MgCl2 H2 Magnesium Hydrochloric acid. Mg2H2Cl---Mg22Cl-H2 Cancel out the Cls because they dont change and your ionic equation is. Post-lab Questions Reacting Magnesium Ribbon with Dilute Hydrochloric acid Solution 1.
Magnesium metal hydrochloric acid rarr magnesium chloride dihydrogen gas Magnesium metal is oxidized to Mg2. Provide the net ionic equation. Magnesium reacts with hydrochloric acid according to the equation.
2HClaq MgCO 3 s MgCl 2 aq H 2 Ol CO 2 g. Mgs 2 HClaq MgCl 2 aq H 2 g This demonstration can be used to illustrate the characteristic reaction of metals with acid a single replacement reaction or to demonstrate the generation of hydrogen gas. The products from the reaction of magnesium hydroxide and hydrochloric acid are magnesium chloride and water.
1 Net Ionic Equations notes worksheet packet Please refer to solubility rules before start writing net ionic equations. Chemistry questions and answers. Mgs 2HClaq rarr MgCl_2aq H_2guarr Are all.
Mgs 2 HClaq MgCl 2aq H 2g This demonstration can be used to illustrate the characteristic reaction of metals with acid a single replacement reaction or to demonstrate the generation of hydrogen gas. First we balance the molecular equa.

Net Ionic Equation For Mg Hcl Magnesium Hydrochloric Acid Youtube
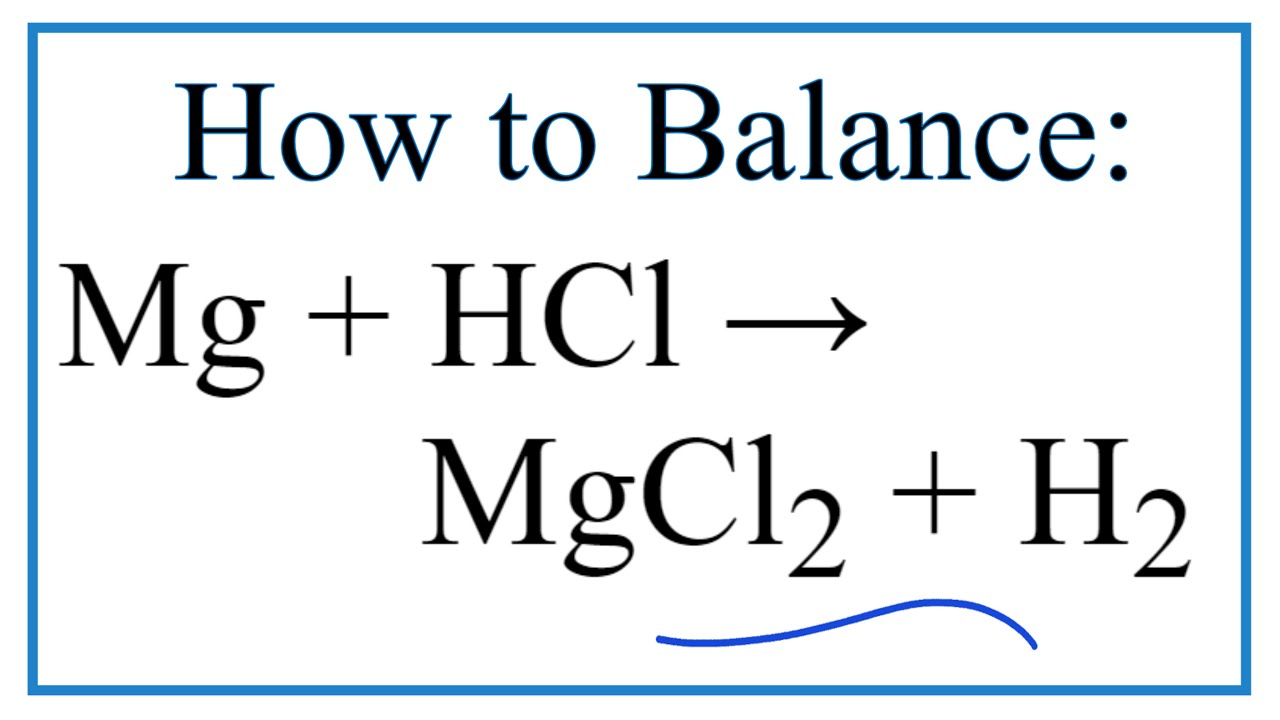
Net Ionic Equation For Mg Hcl Magnesium Hydrochloric Acid Youtube
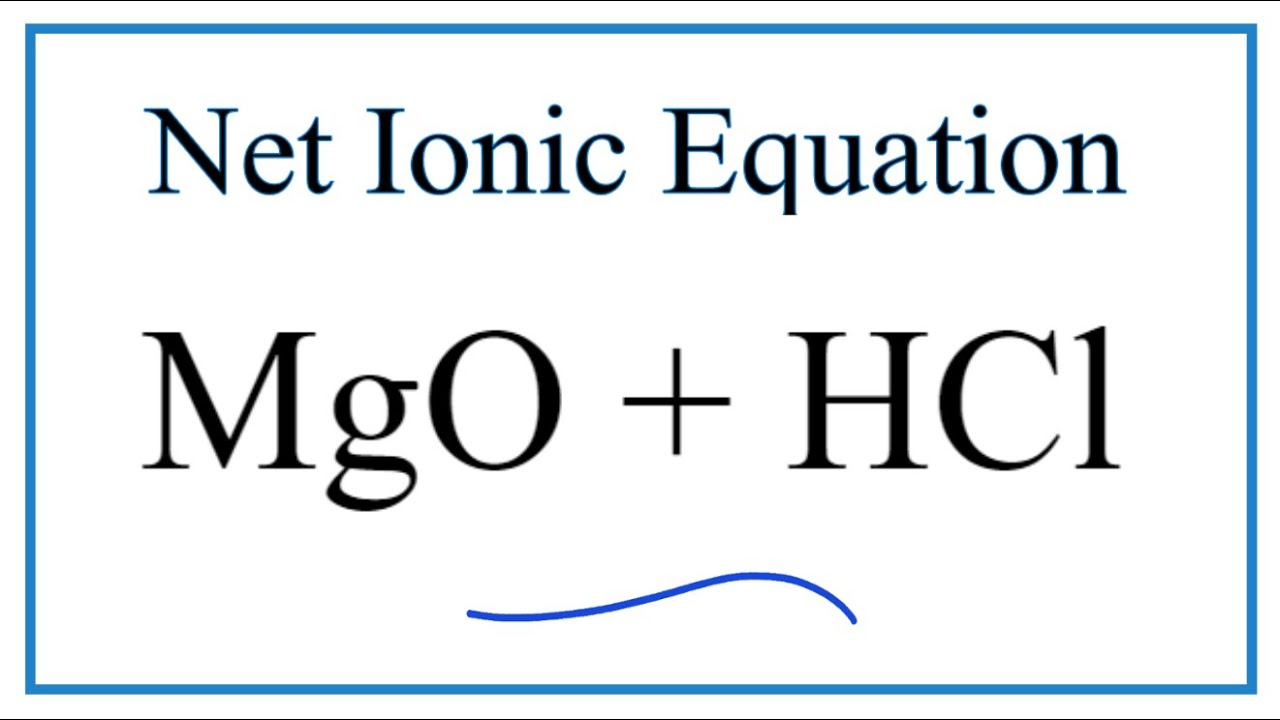
How To Write The Net Ionic Equation For Mgo Hcl Mgcl2 H2o See Note Below Youtube

Acids Alkalis And Neutral Substances Gcse Chemistry Chemistry Tuition Centre

Redox Reactions Half Equations Ionic Equations Teaching Resources

Ionic Equations A Chemical Equation Shows The Number Of Atoms And Molecules Of The Reactants And Products Also Shows Physical State Of Reactants And Products Ppt Download

Ionic Equations A Chemical Equation Shows The Number Of Atoms And Molecules Of The Reactants And Products Also Shows Physical State Of Reactants And Products Ppt Download

How To Determine Formula Of Calcium Chloride Equations Chemical Equation Writing

Chapter 5 Ions And Ionic Compounds What Are The Characteristics Of Ionic Compounds Unit Essential Question Ionic Compound Covalent Bonding Polyatomic Ion

Question Video Writing A Net Ionic Equation For The Reaction Of Solid Calcium Carbonate With A Hydrochloric Acid Solution Nagwa

Solubility Product Constants Silver Chloride Agcl Is Rather Insoluble In Water Careful Experiments Show That If Solid Solubility Silver Chloride Experiments

Acids Alkalis And Neutral Substances Gcse Chemistry Chemistry Tuition Centre

How To Determine Formula Of Calcium Carbonate Equations Chemical Equation Writing

How To Write The Net Ionic Equation For Mg Oh 2 Hcl Mgcl2 H2o Youtube

What Is A Binary Compound Definition And Examples Covalent Bonding Compounds Binary

Bacl Al So Baso Alcl Balanced Equation Barium Chloride Aluminium Sulphate Balanced Equation Youtube Aluminium Sulfate Balance Equation



Comments
Post a Comment